What happens to marine life when a lake or pond is frozen?
 Water is one of the most abundant substance on the surface of the earth. It is the major constituent of almost all life forms and covers about three fourths of the surface of the earth (that's around 70 percent of the earth's surface covered with water - the oceans). Water makes up about 50 - 95 percent of the weight of all living organisms. This means, every living organism needs water to survive, and every living thing is made of at least some amount of water. Thus, without water, there would be no life here on earth or to continue life if it was to become absent. Water in its pure form is a transparent, odorless, tasteless and a neutral substance having a pH of 7 since it is neither acidic or basic. It is also ubiquitous since it can be found dissolved in other substances and the constituent make up of an organism. Water is called the "universal solvent"
Water is one of the most abundant substance on the surface of the earth. It is the major constituent of almost all life forms and covers about three fourths of the surface of the earth (that's around 70 percent of the earth's surface covered with water - the oceans). Water makes up about 50 - 95 percent of the weight of all living organisms. This means, every living organism needs water to survive, and every living thing is made of at least some amount of water. Thus, without water, there would be no life here on earth or to continue life if it was to become absent. Water in its pure form is a transparent, odorless, tasteless and a neutral substance having a pH of 7 since it is neither acidic or basic. It is also ubiquitous since it can be found dissolved in other substances and the constituent make up of an organism. Water is called the "universal solvent" because it dissolves more substances than any other liquid. For example, it is found dissolved in substances such as the soil (containing dissolved minerals), blood and tissue of animals and in plants and their fruit. Apart from needing it to cook our food, have a bath, wash, we need it to regulate and carry out chemical reactions in living things as well as our environment. It is a solvent, a solute, a reactant and a biomolecule. Water is also found in the atmosphere as gas (water vapor) and comes down to the surface of the earth as rain, hail, dew and snow. Water is good but it can be just as harmful. Having no water can cause drought which can lead to famines also having too much water at one given time in our environment can lead to flooding and soil erosion causing death and diseases.
because it dissolves more substances than any other liquid. For example, it is found dissolved in substances such as the soil (containing dissolved minerals), blood and tissue of animals and in plants and their fruit. Apart from needing it to cook our food, have a bath, wash, we need it to regulate and carry out chemical reactions in living things as well as our environment. It is a solvent, a solute, a reactant and a biomolecule. Water is also found in the atmosphere as gas (water vapor) and comes down to the surface of the earth as rain, hail, dew and snow. Water is good but it can be just as harmful. Having no water can cause drought which can lead to famines also having too much water at one given time in our environment can lead to flooding and soil erosion causing death and diseases.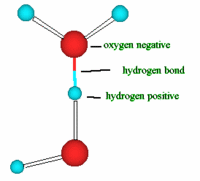 Water has a very simple atomic structure. This structure consists of two hydrogen atoms bonded (attached) to one oxygen atom having the formula H2O. It is the second most common molecule in the Universe after hydrogen (H2). It is the only natural substance that is found as a solid (ice), a liquid and a gas (water vapor) on Earth. Because of this simple composition and structure this gives water very unique physical and chemical properties. This article will not get into detail about forces of attraction between the water (H2O) and its atom but it is important to know that the bonding (attachment) between the hydrogen atom and oxygen atom to form water ( H2O) is called a covalent bonding that is, two hydrogen atoms are joined to a single oxygen atom by single covalent bonds and the
Water has a very simple atomic structure. This structure consists of two hydrogen atoms bonded (attached) to one oxygen atom having the formula H2O. It is the second most common molecule in the Universe after hydrogen (H2). It is the only natural substance that is found as a solid (ice), a liquid and a gas (water vapor) on Earth. Because of this simple composition and structure this gives water very unique physical and chemical properties. This article will not get into detail about forces of attraction between the water (H2O) and its atom but it is important to know that the bonding (attachment) between the hydrogen atom and oxygen atom to form water ( H2O) is called a covalent bonding that is, two hydrogen atoms are joined to a single oxygen atom by single covalent bonds and the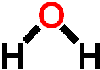 bonding of a hydrogen atom of a water molecule (H2O) to another oxygen atom of a water molecule is called a hydrogen bonding. This bonding takes place because Oxygen is slightly negative and the hydrogen is slightly positive so, the polarity of water molecules results in hydrogen bonding. Thus, this weak attraction is called a hydrogen bond. Hence every water molecule is hydrogen bonded to its four nearest neighbors.
bonding of a hydrogen atom of a water molecule (H2O) to another oxygen atom of a water molecule is called a hydrogen bonding. This bonding takes place because Oxygen is slightly negative and the hydrogen is slightly positive so, the polarity of water molecules results in hydrogen bonding. Thus, this weak attraction is called a hydrogen bond. Hence every water molecule is hydrogen bonded to its four nearest neighbors. During winter some of us based on our geographical location might have noticed a pond or a lake changing in appearance over time from a liquid state on its surface to a solid hard substance called ice. What causes this? Well, as the temperature of water decreases during this time, water becomes more dense. However, when it reaches a temperatures below 4°C, a very unusual thing happens where water begins to expand. This means, the density of water reaches a temperature of 4 degrees below and going below this temperature, the density of water decreases. Above 4 °C it behaves like other liquids; it expands as it warms and contracts when it cools. Water starts to freeze when the temperature approaches 0°C and the molecules no longer move vigorously enough to break their hydrogen bonds. This unusual property of water is what allows ice to float. Because water freezes below 4 degrees C, i.e. at 0 degrees C, ice is less dense than water. The reason for this strange phenomena is that at 4 degrees C, water molecules are packed as tight as they can be and any attempt to push them closer by lowering the temperature, only makes the water molecules repel each other stronger. Water molecules at the freezing point form a crystal lattice structure, (e.g. ice and snow) that is significantly much less dense that liquid water. This means the density of ice is almost ten times lighter than liquid water. This strange property of water is due to the hydrogen bonding in its structure.
During winter some of us based on our geographical location might have noticed a pond or a lake changing in appearance over time from a liquid state on its surface to a solid hard substance called ice. What causes this? Well, as the temperature of water decreases during this time, water becomes more dense. However, when it reaches a temperatures below 4°C, a very unusual thing happens where water begins to expand. This means, the density of water reaches a temperature of 4 degrees below and going below this temperature, the density of water decreases. Above 4 °C it behaves like other liquids; it expands as it warms and contracts when it cools. Water starts to freeze when the temperature approaches 0°C and the molecules no longer move vigorously enough to break their hydrogen bonds. This unusual property of water is what allows ice to float. Because water freezes below 4 degrees C, i.e. at 0 degrees C, ice is less dense than water. The reason for this strange phenomena is that at 4 degrees C, water molecules are packed as tight as they can be and any attempt to push them closer by lowering the temperature, only makes the water molecules repel each other stronger. Water molecules at the freezing point form a crystal lattice structure, (e.g. ice and snow) that is significantly much less dense that liquid water. This means the density of ice is almost ten times lighter than liquid water. This strange property of water is due to the hydrogen bonding in its structure. So, when the surface temperature in a lake or pond reaches 0°C, ice forms and floats on top of the its surface. This ice becomes an insulating layer on the surface by reducing the heat loss from the water below and thus, enabling life to continue in the lake or pond. When ice absorbs enough heat for its temperature to increase above 0°C, the hydrogen bonds can be broken allowing the water molecules to slip closer together (melting). If this strange phenomena did not take place when water turns into ice, then ice would sink to the bottom of lakes and ponds where the marine life would be killed from the ice since it would be formed from the bottom. Also, the ice would not be able to thaw out, since the energy from the air and the sunlight would not be able to penetrate through thick dark layers of ice way below the depth of the pond or lake. So ice being less dense than water will form and float at the surface.
So, when the surface temperature in a lake or pond reaches 0°C, ice forms and floats on top of the its surface. This ice becomes an insulating layer on the surface by reducing the heat loss from the water below and thus, enabling life to continue in the lake or pond. When ice absorbs enough heat for its temperature to increase above 0°C, the hydrogen bonds can be broken allowing the water molecules to slip closer together (melting). If this strange phenomena did not take place when water turns into ice, then ice would sink to the bottom of lakes and ponds where the marine life would be killed from the ice since it would be formed from the bottom. Also, the ice would not be able to thaw out, since the energy from the air and the sunlight would not be able to penetrate through thick dark layers of ice way below the depth of the pond or lake. So ice being less dense than water will form and float at the surface. 32° F (0° C) - 39° F (4° C)
32° F (0° C) - 39° F (4° C)
Therefore, during this season, water in lakes and ponds produce layers of water called strata. This means you will have different temperature levels of water as you move from the top to the bottom of a pond or lake. The top will be much colder so fish, which are cold-blooded, will move to the bottom of ponds or lakes when the water gets too cold, and their metabolism slows down dramatically. Thus using up less energy, which they will need for their survival. So , if it had always been your dream or your hobby to do ice fishing by carving a hole and standing on an ice-covered lake or pond to catch fish, you will now know how it all get started for you to be pulling up a fish from that dark mysterious depths below.
So , if it had always been your dream or your hobby to do ice fishing by carving a hole and standing on an ice-covered lake or pond to catch fish, you will now know how it all get started for you to be pulling up a fish from that dark mysterious depths below.
Did You know also:
 Unlike in freshwater, like lakes and ponds, the presence of salt in the water allows it to get much colder than freshwater before it freezes. Therefore, Arctic and Antarctic fish have developed an antifreeze (proteins) molecules that bind to tiny ice crystals in their blood thus preventing the ice crystals from getting bigger and causing serious cell damage that can lead to death.
Unlike in freshwater, like lakes and ponds, the presence of salt in the water allows it to get much colder than freshwater before it freezes. Therefore, Arctic and Antarctic fish have developed an antifreeze (proteins) molecules that bind to tiny ice crystals in their blood thus preventing the ice crystals from getting bigger and causing serious cell damage that can lead to death.
Conclusion Hence, bodies of water (such as lakes and ponds) freeze from the top down where marine life can be sustained under the ice. If ice did not form on the top of water then polar ice caps would almost not exist since the ice would form from the bottom upward and would take a very long time to reach the surface, if it ever does. Likewise, the world would be a different place if that was so, there would be no: marine life, ice fishing, ice skating, no polar bear, the Antarctic continent would be smaller in size and the list goes on!
Hence, bodies of water (such as lakes and ponds) freeze from the top down where marine life can be sustained under the ice. If ice did not form on the top of water then polar ice caps would almost not exist since the ice would form from the bottom upward and would take a very long time to reach the surface, if it ever does. Likewise, the world would be a different place if that was so, there would be no: marine life, ice fishing, ice skating, no polar bear, the Antarctic continent would be smaller in size and the list goes on!
Related Article:
How Do Fish Breathe?


 R. Edmondson
United States
R. Edmondson
United States











































6 Comments:
The simplest of things yet one of the most important.
Thank you again for a great post.
Have a great week!
Voted too, good luck!
Furkids:
Thank you very much for taking the time out to vote for me!! It is greatly appreciated :). I am really thankful that I made it in the top 15 as a finalist from all the other nominated sites. If I don’t win that’s OK, since making it as a finalist is a great achievement for me and will encourage me to do better.
Thanks once again for everything and catch you later.
Interesting. Cool pic of a water drop.
I voted too, I think.? I just clicked, it that all? Didn't see anything else to click. Let me know if there is something else I should do.
Thanks for the visit and comment :)
Just click the Weblog Award logo box and you will go to the voting area then select "Universal Facts" afer which you click the "vote" button.
Thank you so much for the vote!! I might not win but nevertheless, I am very grateful that I made it in the top 15 as a finalist from all the other nominated sites. This is a great achievement and encouragement for me.
Just want to let everyone know also that they can vote for me again after every 24 hours.
Thanks again.
im 15 and i dont really go to school , you see things like this intorst me more than english or maths, i like learning real things like this , so thanks :)
Post a Comment
<< Home